Chemical Properties
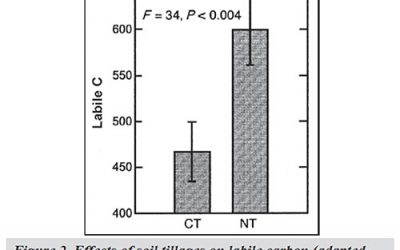
Reactive Carbon
Slaking is the breakdown of large, air-dry soil aggregates (>2-5 mm) into smaller sized microaggregates (<0.25 mm) when they are suddenly immersed in water. Slaking occurs when aggregates are not strong enough to withstand internal stresses caused by rapid water uptake. Internal stresses result from differential swelling of clay particles, trapped and escaping air in soil pores, rapid release of heat during wetting, and the mechanical action of moving water.
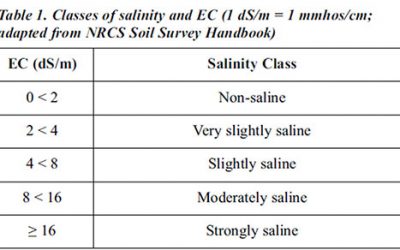
Soil Electrical Conductivity
Soil electrical conductivity (EC) measures the ability of soil water to carry electrical current. Electrical conductivity is an electrolytic process that takes place principally through water-filled pores. Cations (Ca2+, Mg2+, K+, Na+, and NH4 +) and anions (SO4 2-, Cl-, NO3 -, and HCO3 -) from salts dissolved in soil water carry electrical charges and conduct the electrical current.
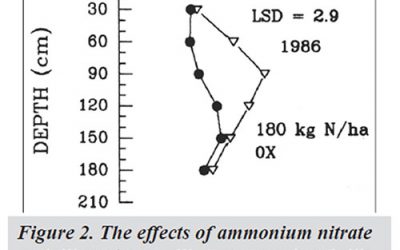
Soil Nitrate
Nitrate (NO3 -) is a form of inorganic nitrogen (N) naturally occurring in soils. Sources of soil NO3 – include decomposing plant residues and animal manure/compost, chemical fertilizers, exudates from living plants, rainfall, and lightning.
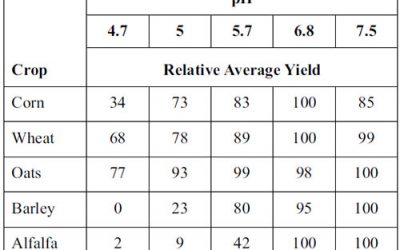
Soil pH
Soil pH generally refers to the degree of soil acidity or alkalinity. Chemically, it is defined as the log10 hydrogen ions (H+) in the soil solution. The pH scale ranges from 0 to 14; a pH of 7 is considered neutral. If pH values are greater than 7, the solution is considered basic or alkaline; if they are below 7, the solution is acidic.

West River Soil Health School Registration Open!
In 2024, the South Dakota Soil Health Coalition will host an additional Soil Health School in west of the Missouri River! The 2024 West River Soil Health School with be held June 26-27 near Caputa, SD! This school will focus on issues specific to the land, climate, and ag production systems of wester South Dakota. Class size is limited, so early registration is strongly encouraged!
News & Events
Farmer reaps higher yields by interseeding soybeans
By Stan Wise Alex Frasier has spent a lot of time studying what it takes to grow a successful crop. After studying ag production and precision technology at Lake Area Technical College, he has worked in ag retail and currently works as an agronomist in Aberdeen, SD....
Farm and ranch innovators to share new ideas at Soil Health Conference
By Stan Wise PIERRE, SD — Before Cooper Hibbard came home to manage his family’s ranch, he studied ag business, rangeland resources and Spanish at California Polytechnic State University and then worked on ranches all over the world. That education and experience...
Wintertime is decision time
By Stan Wise PIERRE, SD – It’s often said that the best time to start improving your land was 20 years ago, but the second-best time is right now. That statement might be harder for ranchers to swallow with winter on their doorstep, nothing growing in their pastures,...


