Soil pH
 Soil pH generally refers to the degree of soil acidity or alkalinity. Chemically, it is defined as the log10 hydrogen ions (H+) in the soil solution. The pH scale ranges from 0 to 14; a pH of 7 is considered neutral. If pH values are greater than 7, the solution is considered basic or alkaline; if they are below 7, the solution is acidic. It is important to recognize that because the pH scale is in logarithmic units, a change of just a few pH units can induce significant changes in the chemical environment and sensitive biological processes. For example, a soil with pH 5 is 10 or 100 times more acidic than a soil with pH 6 or 7, respectively. Sources of H+ ions in soil solution include carbonic acid produced when carbon dioxide (CO2) from decomposing organic matter, root respiration, and the soil atmosphere is dissolved in the soil water. Other sources of H+ ions are root release, reaction of aluminum ions (Al+3) with water, nitrification of ammonium from fertilizers and organic matter mineralization, reaction of sulfur compounds, rainwater, and acid rain. Certain soils are more resistant to a drop or rise in pH (buffering capacity). Therefore, the lime requirement, which is the quantity of limestone (CaCO3) required to raise the pH of an acid soil to a desired pH, must be determined specifically for each field before amending the soil.
Soil pH generally refers to the degree of soil acidity or alkalinity. Chemically, it is defined as the log10 hydrogen ions (H+) in the soil solution. The pH scale ranges from 0 to 14; a pH of 7 is considered neutral. If pH values are greater than 7, the solution is considered basic or alkaline; if they are below 7, the solution is acidic. It is important to recognize that because the pH scale is in logarithmic units, a change of just a few pH units can induce significant changes in the chemical environment and sensitive biological processes. For example, a soil with pH 5 is 10 or 100 times more acidic than a soil with pH 6 or 7, respectively. Sources of H+ ions in soil solution include carbonic acid produced when carbon dioxide (CO2) from decomposing organic matter, root respiration, and the soil atmosphere is dissolved in the soil water. Other sources of H+ ions are root release, reaction of aluminum ions (Al+3) with water, nitrification of ammonium from fertilizers and organic matter mineralization, reaction of sulfur compounds, rainwater, and acid rain. Certain soils are more resistant to a drop or rise in pH (buffering capacity). Therefore, the lime requirement, which is the quantity of limestone (CaCO3) required to raise the pH of an acid soil to a desired pH, must be determined specifically for each field before amending the soil.Relationship to Soil Function
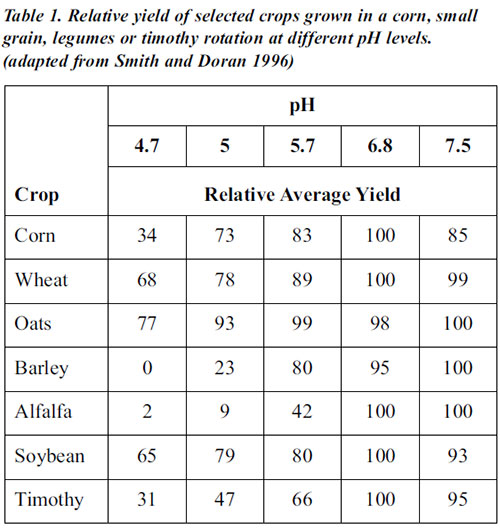
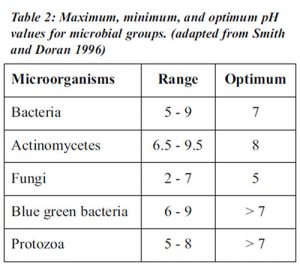 Soil pH affects the soil’s physical, chemical, and biological properties and processes, as well as plant growth. The nutrition, growth, and yields of most crops decrease where pH is low and increase as pH rises to an optimum level (see table 1). Many crops grow best if pH is close to neutral (pH 6 to 7.5) although a few crops prefer acid or alkaline soils. In acid soils, calcium and magnesium, nitrate-nitrogen, phosphorus, boron, and molybdenum are deficient, whereas aluminum and manganese are abundant, sometimes at levels toxic to some plants. Phosphorus, iron, copper, zinc, and boron are frequently deficient in very alkaline soils. Bacterial populations and activity decline at low pH levels, whereas fungi adapt to a large range of pH (acidic and alkaline). Most microorganisms have an optimum pH range for survival and function (see table 2). At very acid or alkaline pH levels, organic matter mineralization is slowed down or stopped because of poor microbial activity linked to bacteria. Nitrification and nitrogen fixation are also inhibited by low pH. The mobility and degradation of herbicides and insecticides, and the solubility of heavy metals are pH dependent. The effects of soil pH on cation availability influence aggregate stability since multivalent cations, such as calcium ions, act as bridges between organic colloids and clays. Some diseases thrive when the soil is alkaline or acidic. Take-all, which is caused by the fungus Gaeumannomyces graminis, is favored by alkaline pH and infects wheat, barley, rye, and several grasses.
Soil pH affects the soil’s physical, chemical, and biological properties and processes, as well as plant growth. The nutrition, growth, and yields of most crops decrease where pH is low and increase as pH rises to an optimum level (see table 1). Many crops grow best if pH is close to neutral (pH 6 to 7.5) although a few crops prefer acid or alkaline soils. In acid soils, calcium and magnesium, nitrate-nitrogen, phosphorus, boron, and molybdenum are deficient, whereas aluminum and manganese are abundant, sometimes at levels toxic to some plants. Phosphorus, iron, copper, zinc, and boron are frequently deficient in very alkaline soils. Bacterial populations and activity decline at low pH levels, whereas fungi adapt to a large range of pH (acidic and alkaline). Most microorganisms have an optimum pH range for survival and function (see table 2). At very acid or alkaline pH levels, organic matter mineralization is slowed down or stopped because of poor microbial activity linked to bacteria. Nitrification and nitrogen fixation are also inhibited by low pH. The mobility and degradation of herbicides and insecticides, and the solubility of heavy metals are pH dependent. The effects of soil pH on cation availability influence aggregate stability since multivalent cations, such as calcium ions, act as bridges between organic colloids and clays. Some diseases thrive when the soil is alkaline or acidic. Take-all, which is caused by the fungus Gaeumannomyces graminis, is favored by alkaline pH and infects wheat, barley, rye, and several grasses.
Problems with Poor Soil pH Levels
Deficiencies of many nutrients, decline of microbial activity and crop yield, and deterioration of environmental conditions are associated with pH levels as discussed in the previous section.
Improving Soil pH
Liming, addition of organic residues rich in basic cations, and crop rotation to interrupt the acidifying effect of leguminous crops increase soil pH. Applying ammonium based fertilizers, urea, sulfur/ferrous sulfate, irrigating with acidifying fertilizers, or using acidifying residues (acid moss, pine needles, sawdust) decrease soil pH. Increasing organic matter increases buffering capacity.
This Page Was Created Utilizing Text And Images From These Sources:
Soil PH, Soil Quality Indicators Fact Sheet- USDA Natural Resources Conservation Service

West River Soil Health School Registration Open!
In 2024, the South Dakota Soil Health Coalition will host an additional Soil Health School in west of the Missouri River! The 2024 West River Soil Health School with be held June 26-27 near Caputa, SD! This school will focus on issues specific to the land, climate, and ag production systems of wester South Dakota. Class size is limited, so early registration is strongly encouraged!
News & Events
Farmer reaps higher yields by interseeding soybeans
By Stan Wise Alex Frasier has spent a lot of time studying what it takes to grow a successful crop. After studying ag production and precision technology at Lake Area Technical College, he has worked in ag retail and currently works as an agronomist in Aberdeen, SD....
Farm and ranch innovators to share new ideas at Soil Health Conference
By Stan Wise PIERRE, SD — Before Cooper Hibbard came home to manage his family’s ranch, he studied ag business, rangeland resources and Spanish at California Polytechnic State University and then worked on ranches all over the world. That education and experience...
Wintertime is decision time
By Stan Wise PIERRE, SD – It’s often said that the best time to start improving your land was 20 years ago, but the second-best time is right now. That statement might be harder for ranchers to swallow with winter on their doorstep, nothing growing in their pastures,...


